Surface stratification determines the interfacial water structure of simple electrolyte solutions
Abstract
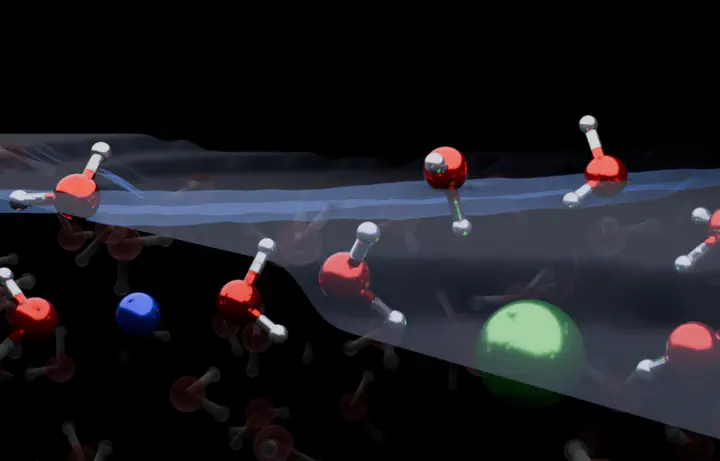
The distribution of ions at the air/water interface plays a decisive role in many natural processes. Several studies have reported that larger ions tend to be surface-active, implying ions are located on top of the water surface, thereby inducing electric fields that determine the interfacial water structure. Here we challenge this view by combining surface-specific heterodyne-detected vibrational sum-frequency generation with neural network-assisted ab initio molecular dynamics simulations. Our results show that ions in typical electrolyte solutions are, in fact, located in a subsurface region, leading to a stratification of such interfaces into two distinctive water layers. The outermost surface is ion-depleted, and the subsurface layer is ion-enriched. This surface stratification is a key element in explaining the ion-induced water reorganization at the outermost air/water interface.